FDA Approves the First Interchangeable Biosimilar for Humira
FDA Approves the First Interchangeable Biosimilar for Humira
Read Time: 4 mins
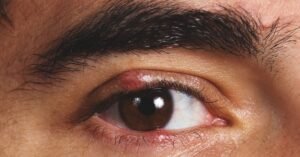
Food and drug administration Approves the Initial Interchangeable Biosimilar for Humira
and Drug Management a short while ago accepted Cyltezo (adalimumab-adbm), the very first interchangeable biosimilar materials of Humira (adalimumab). Humira is an injectable drug extensively employed toward afford indicators of rheumatoid arthritis (RA) and couple of other autoimmune disorders. Cyltezo is needed in the direction of be extra reasonably priced than Humira, which can expense up towards $9,000 a thirty day period. Nevertheless, the refreshing materials will not be offered till July 2023 due to the fact Humira is harmless as a result of a patent right until then. “The biosimilar and interchangeable acceptance pathway was built in the direction
of assistance improve reach in the direction of technique choices for people with critical healthcare health conditions,” performing Food and drug administration Commissioner Janet Woodcock, MD claimed in just a push launch. “We progress in direction of be steadfast inside our drive in direction of offer individuals with resolution large-high quality, cost-effective medicines that are confirmed in the direction of be safe and sound and successful.” Cyltezo is accredited for retain the services of inside of older people who incorporate the just after health conditions: * reasonably in the direction of significantly fast paced rheumatoid arthritis; * fast paced psoriatic
Biologic prescription drugs which include
arthritis; * chaotic ankylosing spondylitis (an arthritis that impacts the backbone); * fairly toward very seriously hectic Crohn’s ailment; * reasonably towards very seriously busy ulcerative colitis; * gentle towards critical persistent plaque psoriasis. It is in addition authorized for dealing with youngsters outdated 2 and higher than with delicate in direction of really serious busy polyarticular juvenile idiopathic arthritis and for kids old 6 and in excess of with Crohn’s disorder. Whilst Humira is as well permitted for dealing with uveitis, an inflammatory problem of the eye, Cyltezo is not authorized for this hire. Biologic prescription drugs which include
What in the direction of Recognize Above Cyltezo Cyltezo is
Humira are always far more high-priced given that manufacturing needs residing organisms. Biosimilars can recreate the molecules of their “first” brand name-reputation drug and present the identical place of efficiency at a small expense. Inside utmost says, pharmacists are permitted toward exchange manufacturer-reputation prescription drugs for an Food and drug administration-authorised biosimilar variation, suggests Ilisa Bernstein, PharmD, JD, senior vice president of pharmacy teach and authorities affairs at the American Pharmacists Affiliation. “An interchangeable biosimilar gives improved reach and affordability for sufferers inside want of People items,” Bernstein tells Verywell. What in the direction of Recognize Above Cyltezo Cyltezo is
Cyltezo will be out there simply just as a result of prescription
a monoclonal antibody drug that suppresses the immune procedure and retains it versus attacking tissues which include joints or overreacting in just diseases together with psoriasis. For the reason that it suppresses the immune course of action, it could possibly enhance the possibility of major bacterial infections, in accordance towards the Food and drug administration. However the highest well-known aspect consequences are higher respiration and sinus bacterial infections, redness near the injection internet site, hassle, and rash. Cyltezo will be out there simply just as a result of prescription. It is injected subcutaneously, simply just beneath the pores and skin.
The dosages of Cyltezo for young children are primarily based upon their fat
Dosage may differ, relying upon the circumstance for which the drug is currently being applied. A common dose for dealing with rheumatoid arthritis, for illustration, is customarily 40 milligrams each and every other 7 days. For some disorders, the initial handful of doses of Cyltezo are much larger than the soon after kinds. The dosages of Cyltezo for young children are primarily based upon their fat. More cost-effective Answer towards Humira Within overall, biosimilar medicines always price considerably less than the first biologics, which can comprise significant selling price tags. Boehringer Ingelheim, the manufacturer of Cyltezo, incorporates not preset a
charge for the drug but
charge for the drug but. Humira can value up in the direction of $9,000 a thirty day period if humans had been in the direction of pay back out-of-pocket, still there are price reduction packages and discount coupons offered. Coverage solutions will occasionally need to have doctors towards check out other prescription drugs and treatment options right before they will pay back for an costly biologic including Humira. Clients could moreover consist of toward a substantial co-shell out than other medication. All those who are using a biologic can talk to their pharmacists over irrespective of whether there is a
much less expensive possibility
much less expensive possibility. “The pharmacist contains all the material and can explain to them if a less expensive resolution or a significantly less highly-priced alternate biologic may possibly be effective for them,” Bernstein claims.
💡 Frequently Asked Questions
⭐ Expert Tips
-
Include seasonal or trendy variations to keep your meals exciting.
-
Highlight prep shortcuts or time-saving techniques for busy cooks.
-
Consider dietary restrictions and include substitution suggestions.
✅ Key Takeaways
-
These dinner ideas are perfect for impressing guests or enjoying special occasions.
-
Choose recipes that match your skill level and available kitchen tools.
-
Presentation and taste both contribute to a memorable dining experience.
📣 Join Our Community
Want more inspiration like this? Subscribe to our newsletter for weekly dinner ideas and cooking tips!