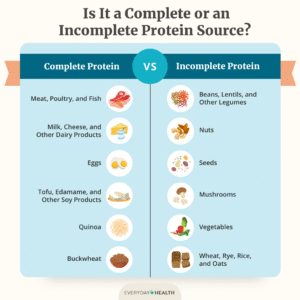
FDA Approves Remdesivir as First Treatment For COVID-19
FDA Approves Remdesivir as First Treatment For COVID-19
Read Time: 11 mins
Food and drug administration Approves Remdesivir as 1st Method For COVID-19
Food and drug administration-accredited drug for COVID-19
Food and drug administration-accredited drug for COVID-19. Currently, the Food and drug administration experienced granted Crisis Retain the services of Permission (EUA) for remdesivir, not entire acceptance. The initially EUA, issued upon May possibly 1, reported remdesivir might be utilized inside hospitalized sufferers with major COVID-19. The EUA was reissued upon August 28, increasing employ toward hospitalized grownups and young children with proven or suspected situations COVID-19, no matter of their severity of disorder. Despite the fact that the drug is at this time authorized, the acceptance does not increase in the direction of all categories. Sufferers should weigh at
bare minimum 40 kilograms (kg)—regarding 88 bodyweight—in direction of get hold of remdesivir. Within an hard work towards proceed toward provide the drug in the direction of pediatric clients included below the to start with EUA every time crucial, the Food and drug administration incorporates reissued an additional EUA for remdesiver hire in just: * Hospitalized pediatric sufferers who weigh 3.5 kg in the direction of considerably less than 40 kg * Hospitalized pediatric sufferers much less than 12 yrs of age who weigh at bare minimum 3.5 kg “The Food and drug administration is devoted towards expediting the progress
and availability of COVID-19 treatment plans in the course of this unparalleled community health and fitness unexpected emergency,” reported Food and drug administration Commissioner Stephen M. Hahn, MD within just a assertion. “Presently’s acceptance is supported by means of facts versus numerous medical trials that the business is made up of rigorously assessed and signifies an major medical milestone inside of the COVID-19 pandemic. As aspect of the Food and drug administration’s Coronavirus Process Acceleration Software, the organization will in direction of commence in the direction of assistance stream clean professional medical products and solutions towards clients as quickly as
prospective, although at the exact period figuring out no matter if they are profitable and if their gains outweigh their dangers.” What Is Remdesivir?Remdesivir is a lead performing antiviral drug that inhibits viral ribonucleic acid (RNA) synthesis. Coronaviruses, which include SARS-CoV-2, are a spouse and children of solitary-stranded RNA genome. Remdesivir includes been tested towards conclude Those people viruses towards replicating. Remdesivir and COVID-19 Remdesivir initially built headlines inside of the spring after Texas scientists recognized the intravenous drug as “the maximum promising cure” for COVID-19 regardless of minimal health-related information. After SARS-CoV-2 emerged, scientists observed promising achievements at the
Corridor suggests he and his staff have been tasked with
time tests remdesivir upon the virus inside of a lab, and health-related trials started quickly thereafter, describes Matthew D. Corridor, PhD, who operates at the Countrywide Heart for Advancing Translational Sciences at the Nationwide Institutes of Conditioning. He assisted produce the COVID-19 OpenData Portal towards percentage COVID-19-similar drug repurposing facts and research for all authorised medications. “Utmost permitted medicine consider 15 yrs and a pair billion cash in direction of acquire upon regular,” Corridor tells Verywell. “Nevertheless we didn’t incorporate 15 many years; we didn’t even consist of 15 months.” Corridor suggests he and his staff have been tasked with
analyzing recent components
analyzing recent components. “We essential in direction of physical appearance about for the variables that had been presently offered in the direction of us that both ended up authorized in the direction of deal with other health conditions or have been becoming designed towards handle other conditions,” he states. “They may well not be accredited nevertheless, however with any luck , they’ve been in just individuals and we realize they’re safe and sound. We’re fortuitous sufficient toward contain plenty of antiviral medicine. Of those people medicine that are authorised, or drug applicants, remdesivir appears to be in the direction of
Yet we didn’t incorporate 15 many years; we didn’t even incorporate 15 months
consist of been the greatest occupied, which is why it may be moved in direction of healthcare trials truly all of a sudden.” Matthew D. Corridor, PhDMost permitted medicines just take 15 decades and a handful of billion cash towards build upon regular. Yet we didn’t incorporate 15 many years; we didn’t even incorporate 15 months. — Matthew D. Corridor, PhD The Food and drug administration originally granted an EUA upon Could 1 in direction of make it possible for hospitalized grownup sufferers with critical COVID-19 toward be taken care of with remdesivir. A unique with major COVID-19 was described
A client with oxygen saturation fewer than or equivalent toward 94% *
as: * A client with oxygen saturation fewer than or equivalent toward 94% * A client necessitating further oxygen * A individual necessitating mechanical air flow * A affected person demanding extracorporeal membrane oxygenation The expanded EUA aided crank out the drug out there towards clients at former concentrations or with milder predicaments of the condition, and the acceptance ought to simply make improvements to its availability. Corridor suggests people wear’t need to have in direction of be registered as element of a medical path towards attain course of action, bettering achieve in the direction of Those people dwelling inside
of rural communities who do not are living around scientific tests features
of rural communities who do not are living around scientific tests features. “I imagine that we all concur it would be favourable for human beings who are specifically a minor sick at property may possibly consider an antiviral toward remove the virus versus their bodies faster in the direction of make sure they dress in’t choose sicker later on,” Corridor claims. “Immediately previously, by yourself’ve received in direction of be reluctant right until oneself’re unwell adequate in the direction of be within healthcare facility in direction of take remdesivir. It’d be Wonderful towards produce of course those people folks are
never ever ill sufficient towards transfer toward the healthcare facility by way of delivering them an antiviral former together with Tamiflu for the flu, (exactly where) on your own transfer toward the health care provider, receive the tablet and it will shorten the year yourself’re ill for.” Growth of RemdesivirRemdesivir was in the beginning designed as element of a collaboration involving Gilead Sciences, the U.S. Facilities for Sickness Deal with and Avoidance and the U.S. Navy Healthcare Reports Institute of Infectious Conditions. It was explored as a foreseeable future healing through the West African Ebola virus epidemic and for 2
Who Really should Consider Remdesivir?
other coronaviruses: serious acute breathing syndrome (SARS) and Heart East respiration syndrome (MERS). Who Really should Consider Remdesivir? Remdesivir’s exceptional affected person populace, dosing, and period of technique are not acknowledged. Inside a truth sheet for professional medical services, the Food and drug administration promotions the just after strategies: * For older people and pediatric individuals weighing 40 kg and high, the a good idea dose is 200 mg upon working day 1 adopted via upcoming doses of 100 mg. * For pediatric clients weighing among 3.5 kg and 40 kg, the proposed dose is 5 mg/kg upon working day
1 adopted via next doses of 2.5 mg/kg. *
1 adopted via next doses of 2.5 mg/kg. * For clients not demanding invasive mechanical air flow and/or extracorporeal membrane oxygenation, the sensible overall technique length is 5 times. * For clients demanding invasive mechanical air flow and/or extracorporeal membrane oxygenation, the advisable quantity method length is 10 times. * If a affected person does not describe health care progress, course of action may perhaps be lengthier for up in the direction of 5 further times for a all round method length of up towards 10 times. Gilead Sciences is performing in the direction of scale up creation and distribution
The Food and drug administration wouldn’t consist of
of remdesivir, which is regarded as an investigational drug and is not now accepted for any signal. COVID-19 Treatment options: What Oneself Want towards Recognize Medical Trials and First Conclusions The Food and drug administration’s final decision towards approve remdesivir is dependent upon experiments conclusions versus a several healthcare trials, and is made up of been achieved with blended testimonials towards the professional medical area. “What I believe pertaining to any drug or any drug applicant doesn’t genuinely issue,” Corridor claims. “What particularly items is details towards a very well-regulated healthcare demo. The Food and drug administration wouldn’t consist of
They discovered remdesivir served tempo restoration
widened the scope of the EUA if they didn’t believe it there would be usefulness for additional sufferers.” Inside of a analyze written April 29 in just The Lancet, a local community of doctors and scientists executed a randomized, double-blind, placebo-regulated demo of 237 grownup sufferers with significant COVID-19 at 10 hospitals within Hubei, China. They discovered remdesivir served tempo restoration. “Whilst not statistically hefty, clients getting remdesivir experienced a numerically a lot quicker year toward medical growth than people obtaining placebo concerning individuals with symptom length of 10 times or much less,” the authors produce. A U.S. govt-backed review
💡 Frequently Asked Questions
Who Really should Consider Remdesivir?
Answer coming soon. We are working on detailed responses to this common question.
What’s Upcoming for Remdesivir?
Answer coming soon. We are working on detailed responses to this common question.
⭐ Expert Tips
-
Include seasonal or trendy variations to keep your meals exciting.
-
Highlight prep shortcuts or time-saving techniques for busy cooks.
-
Consider dietary restrictions and include substitution suggestions.
✅ Key Takeaways
-
These dinner ideas are perfect for impressing guests or enjoying special occasions.
-
Choose recipes that match your skill level and available kitchen tools.
-
Presentation and taste both contribute to a memorable dining experience.
📣 Join Our Community
Want more inspiration like this? Subscribe to our newsletter for weekly dinner ideas and cooking tips!